PharmaTrace has been approved for 300,000 HBAR in funding from the Thrive Hedera program to bring a next‑generation DePIN track‑and‑trace platform for global pharma supply chains onto Hedera. This milestone validates PharmaTrace’s hybrid public‑private architecture and signals growing institutional trust in regulated DePIN infrastructure for healthcare.
For years, pharma and healthcare manufacturers, CMOs, packagers, and distributors have been told that “blockchain will fix traceability,” only to run into closed pilots, slow networks, or compliance dead ends. PharmaTrace’s selection for 300,000 HBAR in Thrive Hedera funding is a concrete signal that the market is shifting from proofs‑of‑concept to production‑grade, regulation‑ready infrastructure.
Being backed by a Hedera ecosystem program positions PharmaTrace not as a speculative experiment, but as a core DePIN layer for verifiable, compliant supply chain events that regulators, brand owners, and patients can rely on.
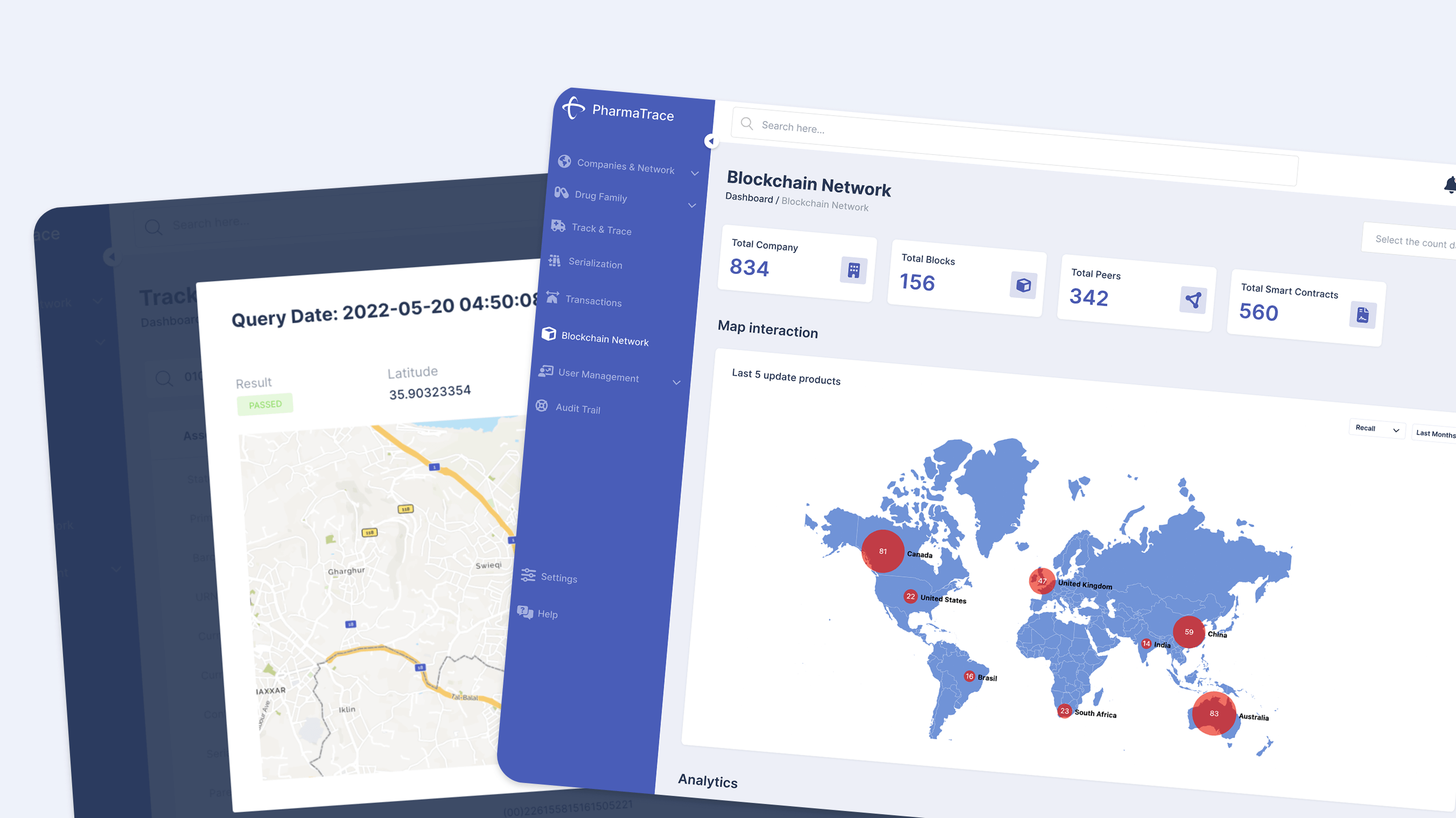
PharmaTrace is an enterprise‑grade track‑and‑trace DePIN platform, already live with a number of pharma and healthcare clients, focused on serialization, anti‑counterfeiting, and regulatory compliance. The platform today runs on a Hyperledger Fabric permissioned network, optimized for privacy and performance in regulated environments.
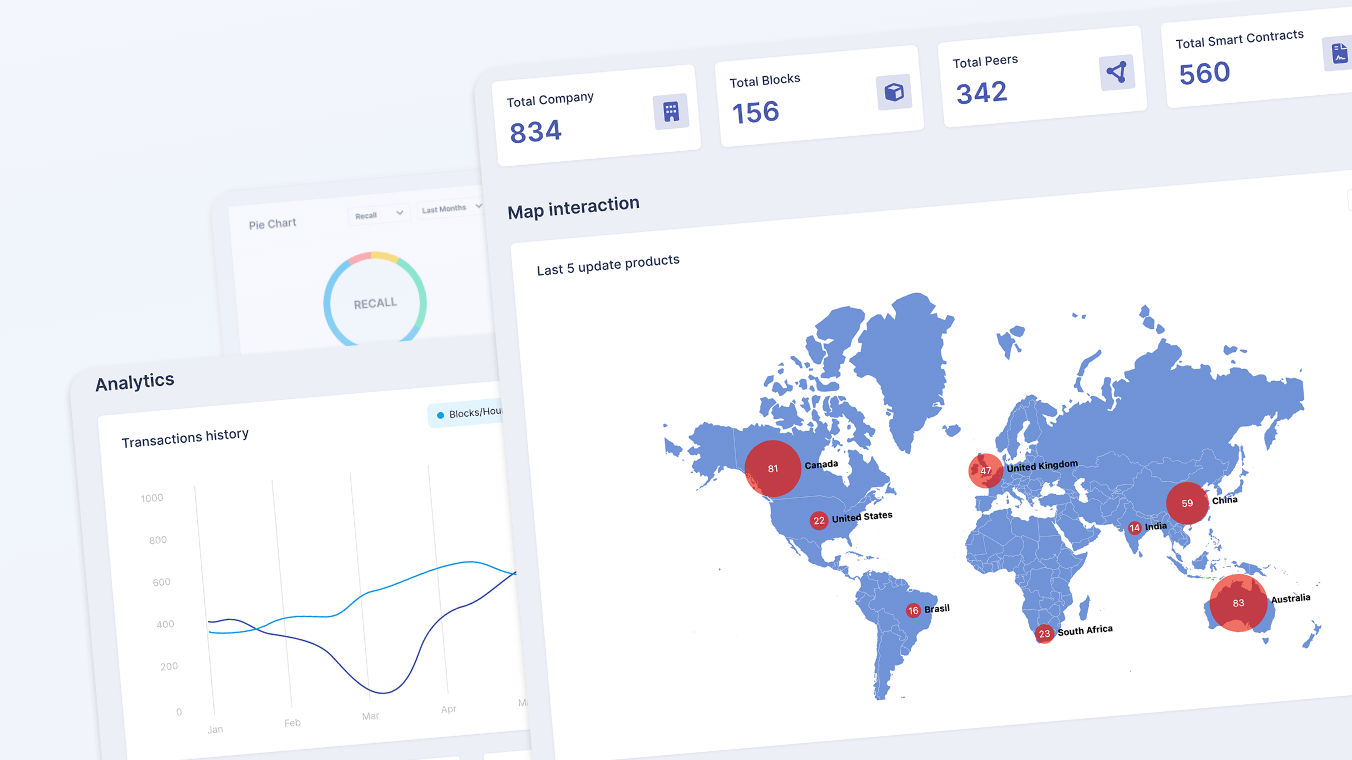
The next phase is a controlled migration to a public‑permissioned architecture using Hedera’s services, allowing the network to scale globally without compromising privacy, performance, or regulatory alignment. The approved 300,000 HBAR funding accelerates this transition from a private, siloed ledger to a transparent, interoperable DePIN layer where on‑chain data becomes an asset for manufacturers, regulators, logistics providers, and technology partners.
Hedera’s hashgraph technology offers several advantages that map directly to the demands of pharma and healthcare supply chains:
Equally important, Hedera’s energy‑efficient consensus supports ESG commitments and sustainability reporting, which are increasingly embedded in pharma procurement and compliance frameworks. The hybrid architecture — leveraging Hedera as a public, verifiable layer while preserving sensitive business logic and data inside the existing Hyperledger Fabric modules — removes one of the biggest blockers to blockchain adoption in regulated industries: how to reconcile transparency with privacy.
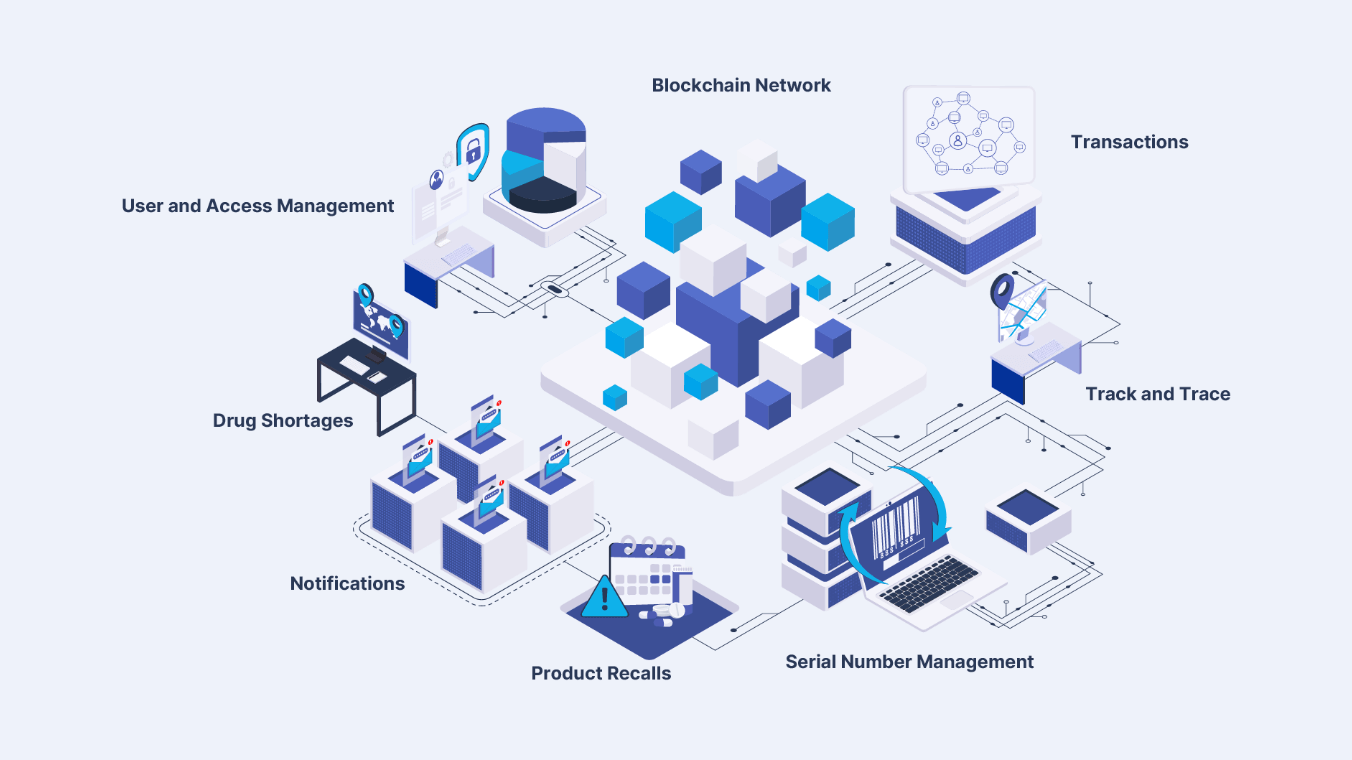
The Hedera integration is designed around specific services that extend what PharmaTrace already delivers to clients:
Mirror nodes and public infrastructure will allow regulators, auditors, and authorized ecosystem partners to independently verify key events without direct access to private systems, strengthening trust while maintaining permissioned data access models. Smart contracts and native interoperability will support advanced use cases such as automated compliance workflows, incentive mechanisms, and cross‑ecosystem integrations.
Enterprise buyers comparing track‑and‑trace and serialization solutions typically evaluate players like TraceLink, Optel, MediLedger, and VeChain. Against this backdrop, PharmaTrace’s Hedera‑powered DePIN approach offers several differentiators:
For customers and partners, that means lower risk, fewer internal disruptions, and a clearer roadmap: keep your validated processes and infrastructure, while gaining a public‑permissioned audit layer, programmable incentives, and access to a growing Hedera ecosystem. For regulators and auditors, it means more transparent, independently verifiable data without forcing confidential commercial details into the open.
As part of the Hedera roadmap, PharmaTrace plans to launch a native utility token that is fully aligned with supply chain realities rather than speculation. This token will:
In time, staking and governance features will give key ecosystem participants a voice in how incentives, upgrades, and new modules evolve—aligning the economic layer with the interests of manufacturers, CMOs, logistics providers, and technology partners instead of purely financial speculation.
Beyond the platform itself, PharmaTrace will actively contribute back to the Hedera developer and DePIN ecosystems by:
Technical documentation, benchmarking results, and playbooks will be shared through official Hedera channels and community hubs, while the team engages in governance votes and ecosystem initiatives. For PharmaTrace customers and partners, this means your platform is not only funded and evolving — it is co‑shaping the standards and infrastructure of the broader Hedera DePIN landscape.
For existing PharmaTrace customers, this announcement is a reassurance: the platform you use today is funded for its next evolution, backed by a leading public network ecosystem, and designed to keep you ahead of regulatory, ESG, and technological change. You gain a clearer, safer route from private, internal tracking to verifiable, ecosystem‑wide traceability and incentives — without compromising privacy or stability.
The Thrive Hedera approval and 300,000 HBAR grant are validation that PharmaTrace is not just another traceability tool, but a DePIN platform with real traction, a realistic roadmap, and alignment with one of the most advanced public‑permissioned networks in the market. PharmaTrace builds the next layer of verifiable, incentive‑driven infrastructure for the global healthcare supply chain on Hedera.